Don’t miss the opportunity to reduce Scope 3 T&L emissions without impacting your supply chain Learn more >
Don’t miss the opportunity to reduce Scope 3 T&L emissions without impacting your supply chain Learn more >
Don’t miss the opportunity to reduce Scope 3 T&L emissions without impacting your supply chain Learn more >
Don’t miss the opportunity to reduce Scope 3 T&L emissions without impacting your supply chain Learn more >

Nationwide warehousing opportunities for freight forward supply chains
Nationwide warehousing opportunities for freight forward supply chains


Real freight experts supporting your supply chain from origin to destination
Real freight experts supporting your supply chain from origin to destination
Discover sustainable transportation & logistics options for international supply chains
Discover sustainable transportation & logistics options for international supply chains
24|7|365 AOG desk for time-critical support to the aviation industry Learn more >
24|7|365 AOG desk for time-critical support to the aviation industry Learn more >
Forced labor compliance for international supply chains
Forced labor compliance for international supply chains


Green is proud to join the Smart Freight Centre (SFC), an advocate for sustainable practices in the freight transportation industry –
This strategic alliance marks Green Worldwide Shipping’s dedicated involvement in three programs led by the Smart Freight Centre — Global Logistics Emissions Council (GLEC), Clean Cargo, and Clean Air Transport.

Green is proud to join the Smart Freight Centre (SFC), an advocate for sustainable practices in the freight transportation industry –
This strategic alliance marks Green Worldwide Shipping’s dedicated involvement in three programs led by the Smart Freight Centre — Global Logistics Emissions Council (GLEC), Clean Cargo, and Clean Air Transport.



Green Worldwide Shipping® joins a consortium of over 20 companies, including giants like Amazon, Electrolux Group, IKEA, and Patagonia, in this historic tender.
The group’s mission is to accelerate the commercial deployment of zero-emissions shipping services, cultivate a competitive market for these services, and combat carbon emissions. What sets the ZEMBA tender apart is its ambitious goal of achieving a minimum 90% reduction in greenhouse gas emissions compared to traditional fossil fuels on a lifecycle basis.
Our phones are answered by real people that love what they do, not automated prompts.
We are real freight experts and can help with any level of transportation & logistics solutions.
We empower our people with the latest technologies, so they can spend more time servicing your business.
We have a sustainable business model ensuring we will be here for generations to come.
When you join the company, you are made to feel welcome.
I’m proud to tell others I work here.
I feel good about the ways we contribute to the community.
I am able to take time off from work when I think it’s necessary.
When I look at what we accomplish, I feel a sense of pride.
Great Place To Work® Certification™ is the most definitive “employer-of-choice” recognition based entirely on what employees report about their workplace experience – specifically, how consistently they experience a high-trust workplace. Great Place to Work Certification is recognized worldwide by employees and employers alike and is the global benchmark for identifying and recognizing outstanding employee experience.
When you join the company, you are made to feel welcome.
I’m proud to tell others I work here.
Great Place To Work® Certification™ is the most definitive “employer-of-choice” recognition based entirely on what employees report about their workplace experience – specifically, how consistently they experience a high-trust workplace. Great Place to Work Certification is recognized worldwide by employees and employers alike and is the global benchmark for identifying and recognizing outstanding employee experience.
I feel good about the ways we contribute to the community.
I am able to take time off from work when I think it’s necessary.
When I look at what we accomplish, I feel a sense of pride.
TRACK YOUR SHIPMENT

Search our articles, blogs, resources, videos & more!
Don’t see the question & answer you’re looking for?
Select the Ask Green button and we’ll answer your question directly!

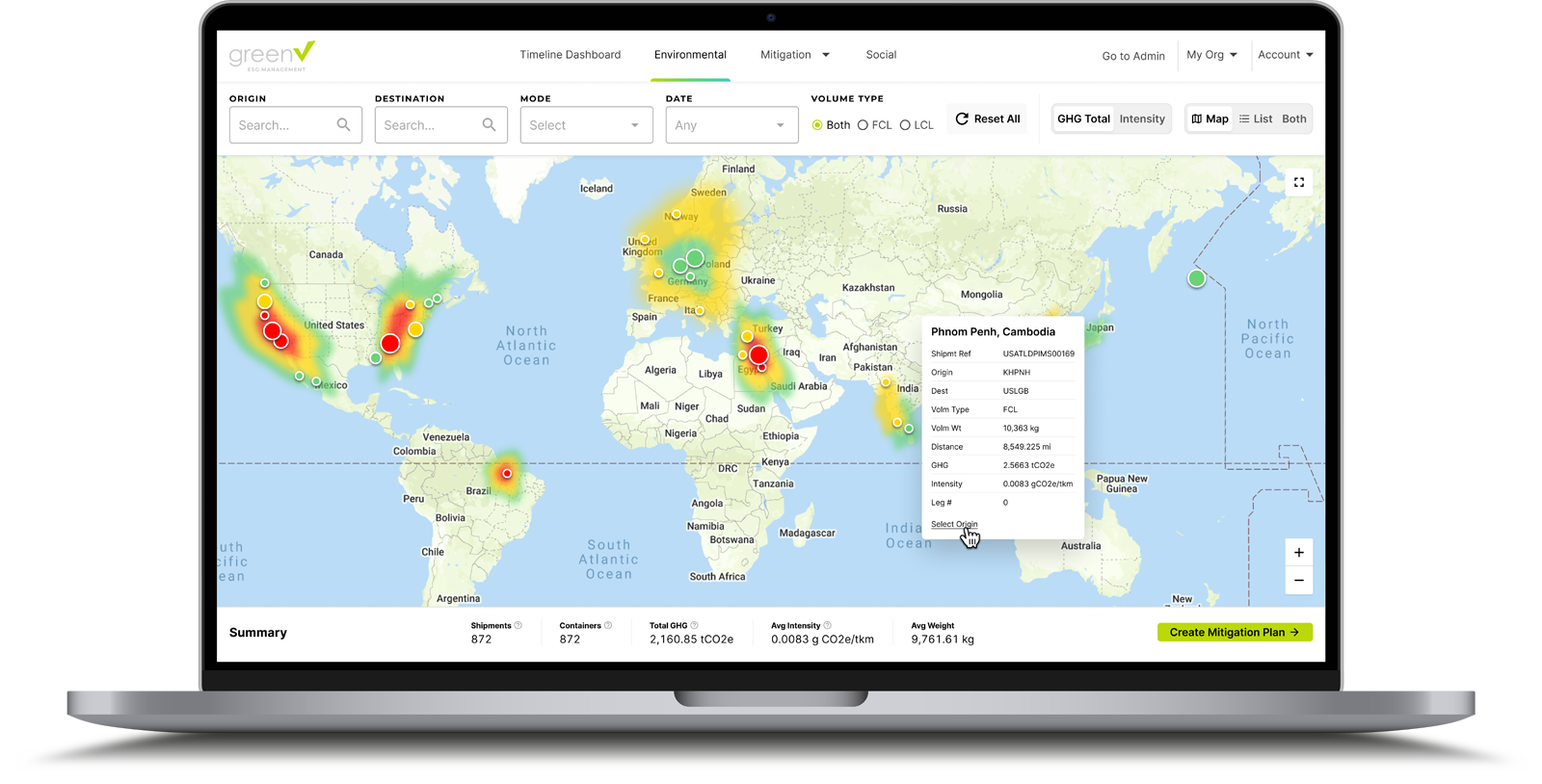
GreenX is a revolutionary shipment control platform that puts real-time transparency back into the hands of logistics professionals. Shippers can rest assured that GreenX is not just an out-of-the-box solution…



GreenX is a revolutionary shipment control platform that puts real-time transparency back into the hands of logistics professionals. Shippers can rest assured that GreenX is not just an out-of-the-box solution…

